C60/oil solution (Lipofullerene) irradiated with UV Light: What happens?
Since the early experimental studies on the solutions of C60 fullerenes in vegetable oils, it was observed that the color of such solutions was not stable at room temperature, changing slowly from the original purple/violet color to magenta and then to brown. This phenomenon was investigated by researchers such as F. Cataldo who published his results in 2010.
C60 dissolved in any kind of unsaturated lipids (or organic solvents such as Cyclohexene) undergoes spectral changes associated to the addition reaction on the fullerene cage. This reaction is triggered by the lipid autoxidation where oxygen bonds to lipid to form a lipid peroxide.
This lipid peroxide attaches to the C60 (which acts as a free radical chain braking agent) and decomposes, leading to a closed cage C60 epoxide with the oxygen being added to a double bond shared by two hexagons (see below).
This reaction is slow at room temperature and the good news is: C60 epoxide formed in solution not only retains its radical scavenging properties but is even enhanced compared to pure C60 (study by Matsubayashi 2008). However, no toxicity data are available for this compound!

Under irradiation with UV light (254nm) in air the oxidation of the C60 molecule is dramatically accelerated (app. 10'000 times faster). After 20 minutes most of the C60 was oxidized. Beside the close cage epoxide (above) another epoxide was found: the open cage [5,6]-oxidoannulene (below).
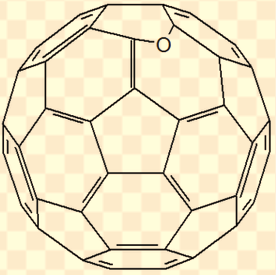
The results of this study suggest to keep the bottles containing C60 dissolved in an vegetable oil (also called lipofullerene) tightly closed in a dark and cool place. Opened bottles should be consumed in a timely manner.
Summary:
We do not recommend to irradiate lipofullerene with UV light in the presence of oxygen. The resulting concotion might still have anti oxidative properties, but it has not been proven to be harmless neither to test animals nor to humans.
